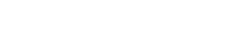InflammaSkin® subcutaneous injection for psoriasis model
Research by C. Jardet (Genoskin), E. Bartnik (Sanofi-Aventis Deutschland), D. Ding-Pfennigdorff (Sanofi-Aventis Deutschland), E. Braun (Genoskin), P. Descargues (Genoskin)
Evaluation of pharmacological responses to subcutaneous injection of adalimumab in InflammaSkin®
a full-thickness human ex vivo skin model reproducing key features of psoriatic lesions

Preclinical skin models closely mimicking inflammatory features in diseased skin such as psoriasis are needed to support the development of anti-inflammatory drugs in dermatology. Approaches based on the incorporation of immune cells in reconstructed skin models are now developed. However, these skin models lack tissue complexity and diversity of immune cells and do not support the delivery of subcutaneously-delivered therapeutic compounds. Genoskin has developed and characterized InflammaSkin®, a T-cell driven skin inflammation model for psoriasis based on the activation and differentiation into Th17/Th1 phenotype of skin resident T cells. This model is composed of epidermis, dermis and adipose tissue, enabling subcutaneous delivery of biologics. In this study, we aimed at assessing pharmacological response of the model to subcutaneous administration of adalimumab.
Production of InflammaSkin® model from healthy ex vivo skin biopsies
HypoSkin® and InflammaSkin® models were produced from healthy ex vivo skin samples of one donor (Female, 67 years old) and cultured in standard cell culture conditions for 7 days.
From day 4 to day 6, 23 μL Betamethasone was topically applied at the surface of skin models.
At day 3, 35 μL Adalimumab at either 5 nM or 50 nM were subcutaneously injected in the hypodermis of the models.
The study was performed on 3 models for each condition.
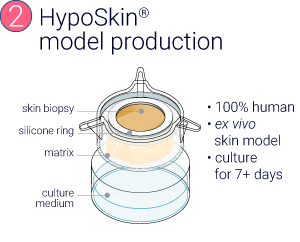
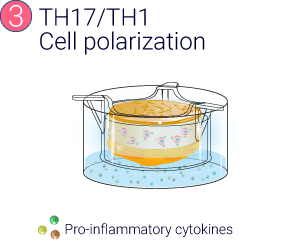
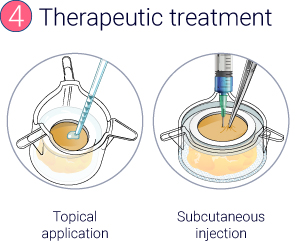
From a healthy skin biopsy to an injectable inflammation assay.
Improvement of skin structure and downregulation of psoriasis-associated markers in a pilot study following adalimumab subcutaneous injection
Hematoxylin and Eosin (H&E) staining was performed on 5 μm thickness paraffin-embedded skin cross-sections. Scale bar 100 μm.
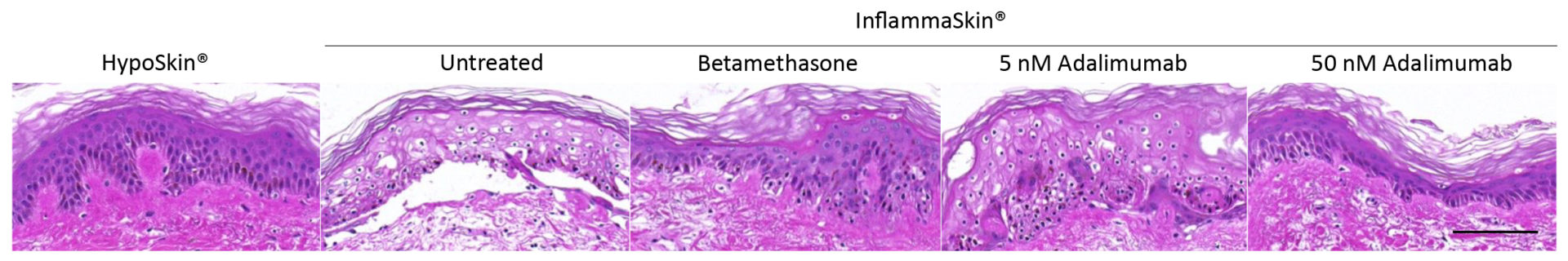
Quantitative analysis of skin structure integrity was performed through attribution of a score depending on defects detected in the models on H&E stained sections:
0: Uncultured skin without any changes
1: Early signs of keratinocytes separation (spongiosis)
2: Advanced separation and volume increase of keratinocytes (hypertrophy) / Early signs of dermis disorganization
3: Nuclei condensation (pyknosis) / Advanced dermis disorganization
4: Cytoplasms vacuolation
5: Stratum basale detachment from dermis / Release of stratum spinosum
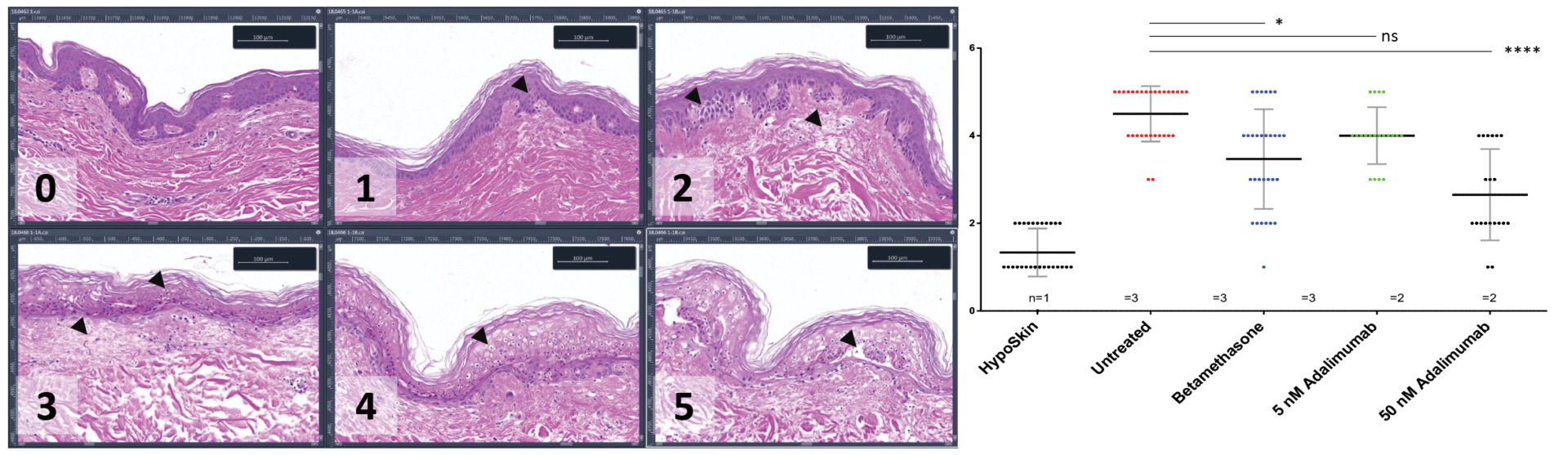
Anti-S100A7 / Keratin 16 (K16) double immunofluorescence staining was performed on 5 μM thickness paraffin-embedded skin cross-sections. For each marker, normalized positive area expressed was quantitatively analyzed. For each condition, single replicates values, mean and standard deviation are presented.
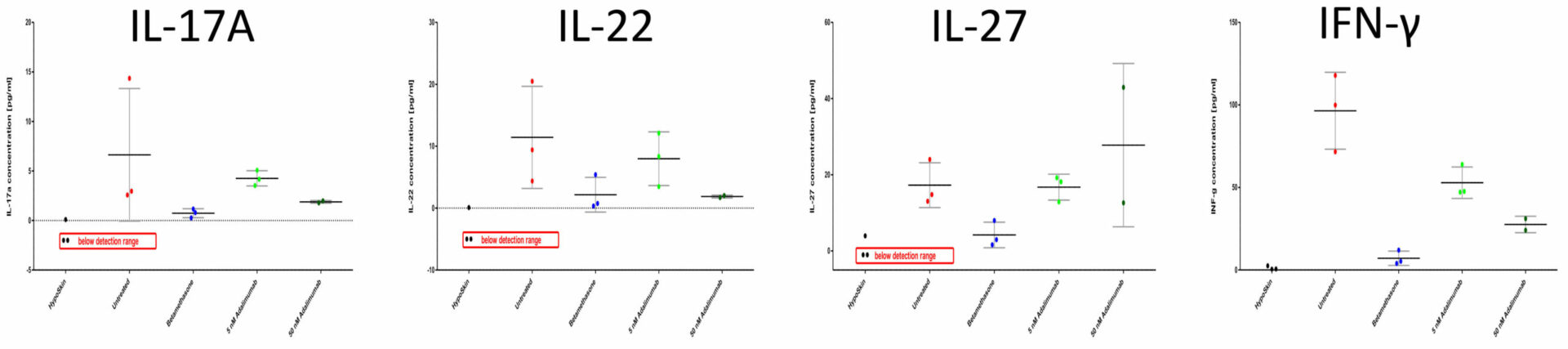
Dose-depending downregulation of IL-17A, IL-22 and INF-γ but not IL-27 release
Levels of cytokines released into the culture media were measured with the Mesoscale (Rockville, USA) human V-Plex Proinflammatory and Th17 Panels. For each analyzed cytokine, concentrations in each replicate, as well as mean values and standard deviation, are presented.