Psoriasis research studies
A new approach to anti-psoriasis drug assessment
Preclinical anti-psoriasis efficacy studies
Innovative anti-psoriasis drug assays for better drug development

Genoskin uses an entirely new approach to help biotech and pharmaceutical companies assess the efficacy of anti-psoriasis compounds and drugs. We combine real human data generation platforms, hi-tech expertise and artificial intelligence to deliver reliable, unbiased, first-in-human data on your anti-psoriasis treatment without actually involving humans. Below you’ll find an overview of why and how we do it.
Reproducible
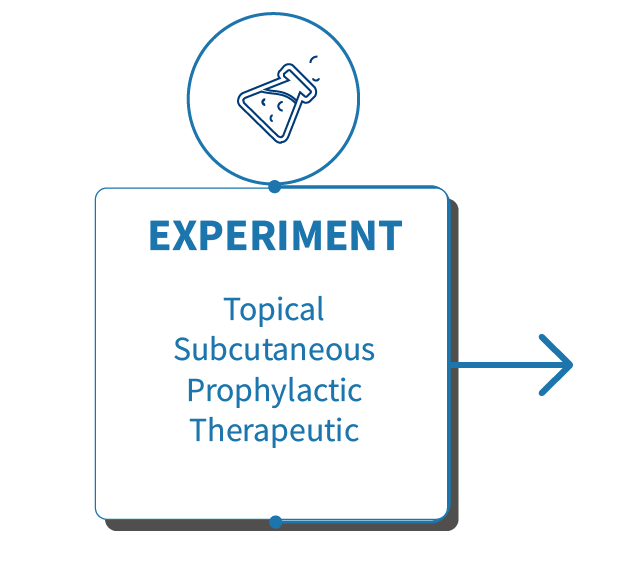
Genoskin’s human data generation platforms are standardized for reproducible results.
T-cell driven
Our anti-psoriasis studies are based on the live response of our unique T-cell driven human psoriasis skin model.
Efficacy
We study efficacy for both biologics and small-molecule drugs and for different administration routes.
Platform
We use cutting-edge AI-based technology to generate in-depth data for prophylactic and therapeutic drugs.
Anti-psoriasis efficacy studies for all types of treatments
High-quality data for prophylactic & therapeutic treatments

To help biotech and pharmaceutical companies assess the efficacy of anti-psoriasis drugs & compounds, Genoskin uses its unique InflammaSkin® ex vivo psoriasis skin model. To this day, this live and immunocompetent human skin model is the only ex vivo skin model to feature T-cell mediated inflammation. Our high-level preclinical drug studies can be conducted for anti-psoriasis treatments that are designed for prophylactic or therapeutic use. For prophylactic treatments, the InflammaSkin® ex vivo human skin model will be treated at day 0 to show efficacy against the onset of the condition. Therapeutic compounds and treatments will only be administered at day 3 to research efficacy.
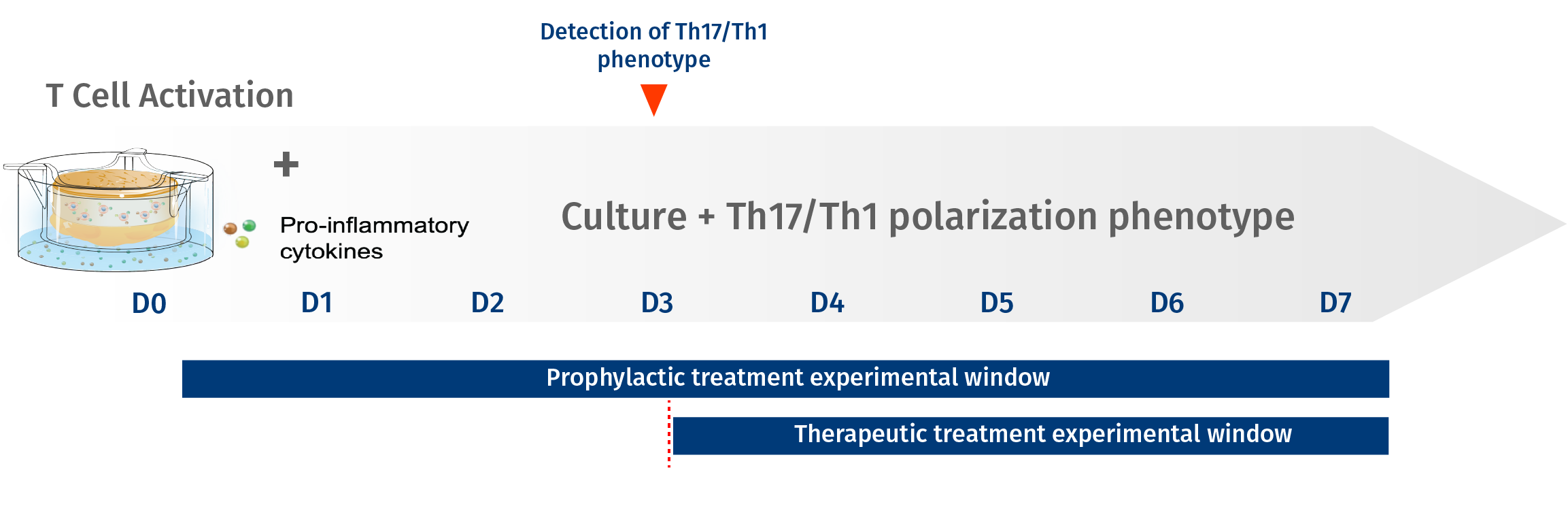
Anti-psoriasis efficacy studies for biologic & conventional drugs
Research the efficacy of all available options
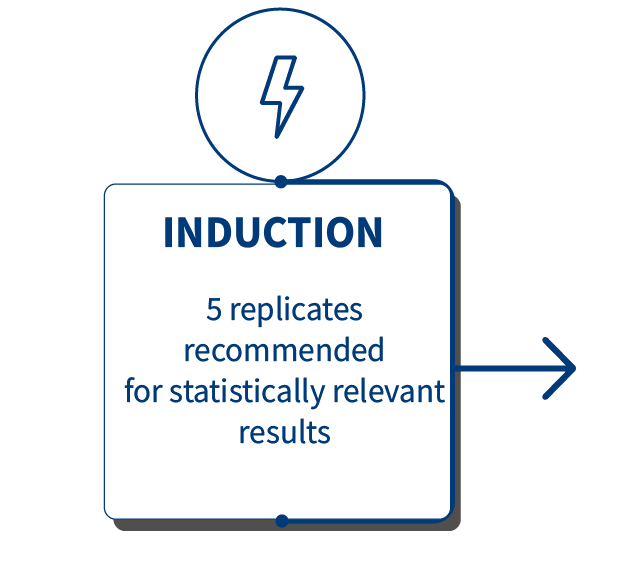
InflammaSkin® psoriasis skin model can be used to assess the efficacy of anti-inflammatory drugs targeting T-cells as well as more specific psoriasis targets such as IL-17, IL-22 and TNF-α. It is also possible to study other targets to fit your needs and requirements. Our InflammaSkin® model is compatible with conventional, small-molecule drugs and has also been validated for biologics to study drug efficacy. Below you’ll see the results of an anti-TNF alfa efficacy study after subcutaneous injection of the Adalimumab biologic.
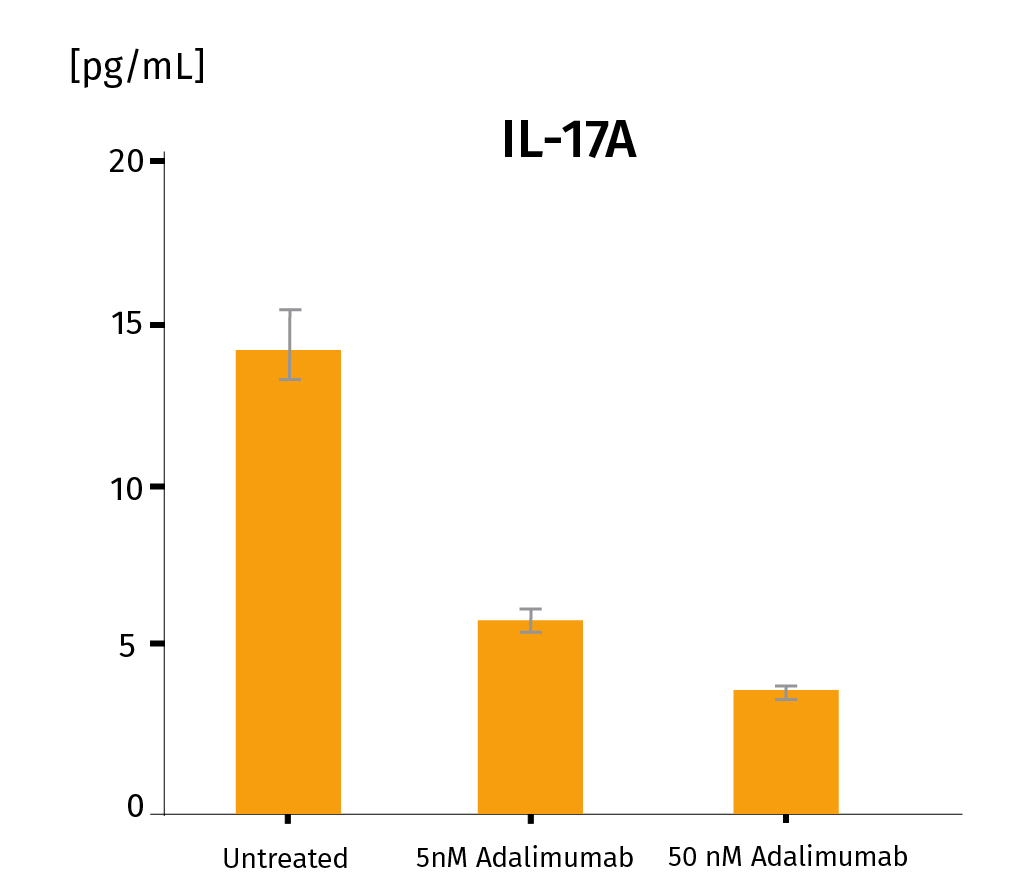
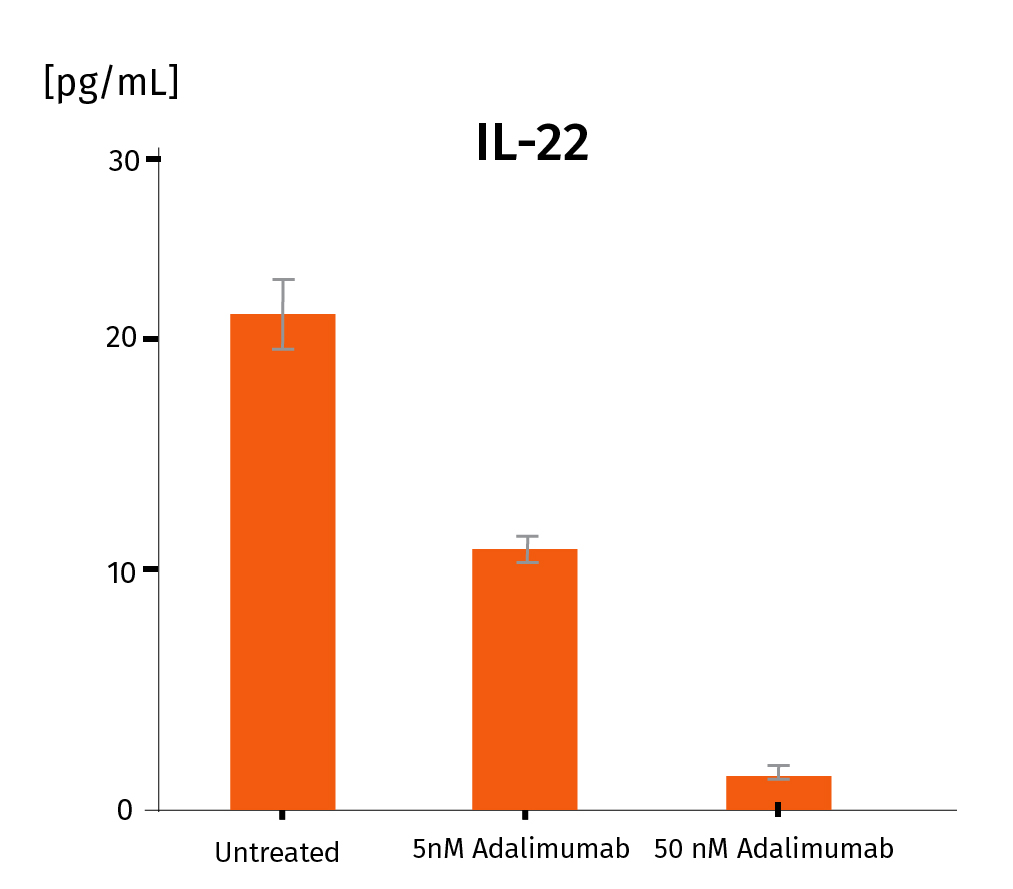
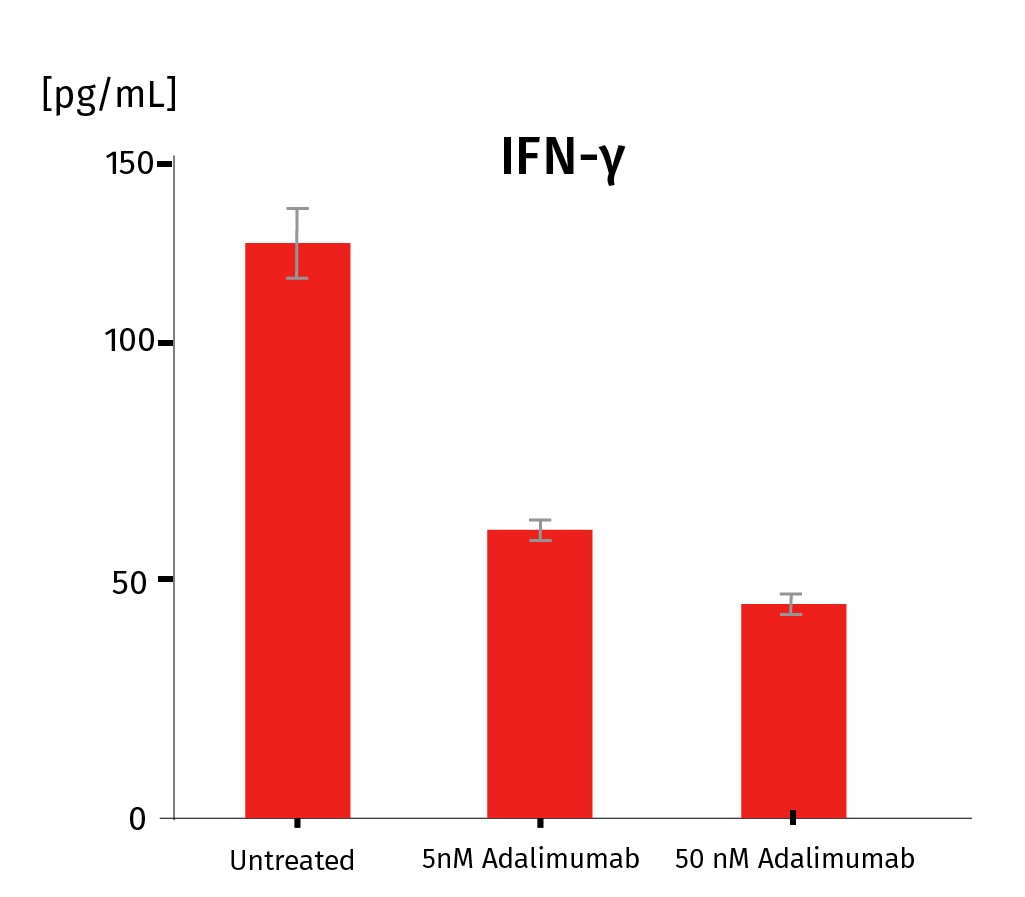
Adalimumab was injected at D3 under 2 conditions : 5nM and 50nM with 3 replicates for each condition. Measurement of psoriasis-relevant cytokines released shows a dose-response down-regulation for IL-17A, IL-22, INF-γ. These results prove the adequacy of the InflammaSkin® platform to assess the efficacy of subcutaneously injectable anti-psoriasis drugs. For more detailed results, don’t hesitate to download the scientific poster.
Anti-psoriasis efficacy studies for multiple administration routes
And discover the best way to deliver your anti-psoriasis treatment

To date, Genoskin is the only company in the world that provides a live ex vivo human skin model that allows testing subcutaneous injection. For the purpose of anti-psoriasis studies, we can therefore conduct efficacy studies after topical or subcutaneous administration. Moreover, our InflammaSkin® platform can also help you define the most efficient administration route for drug delivery.

Subcutaneous injection

Topical application
Below you’ll find the study results on the pharmacological efficacy of two prophylactic anti-inflammatory drugs: Betamethasone Dipropionate (BDP gel) and a PDE4 inhibitor cream, which were applied topically on a daily basis, from D0 to D6.
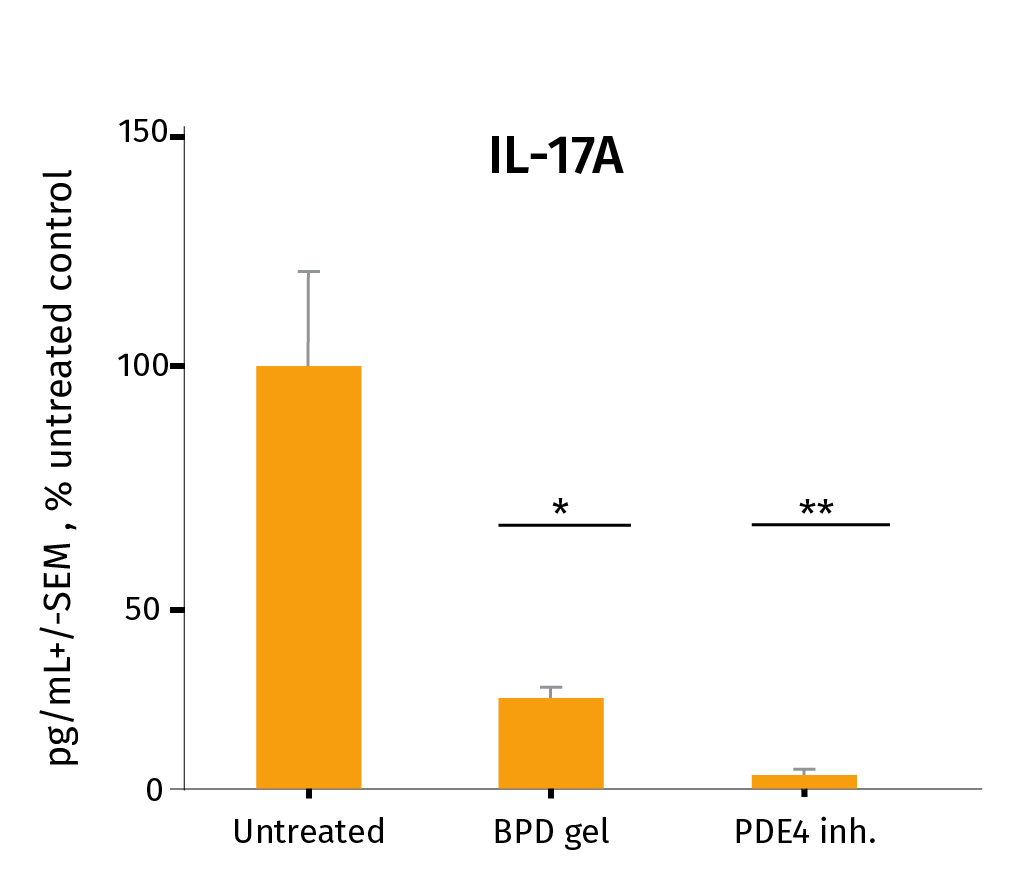
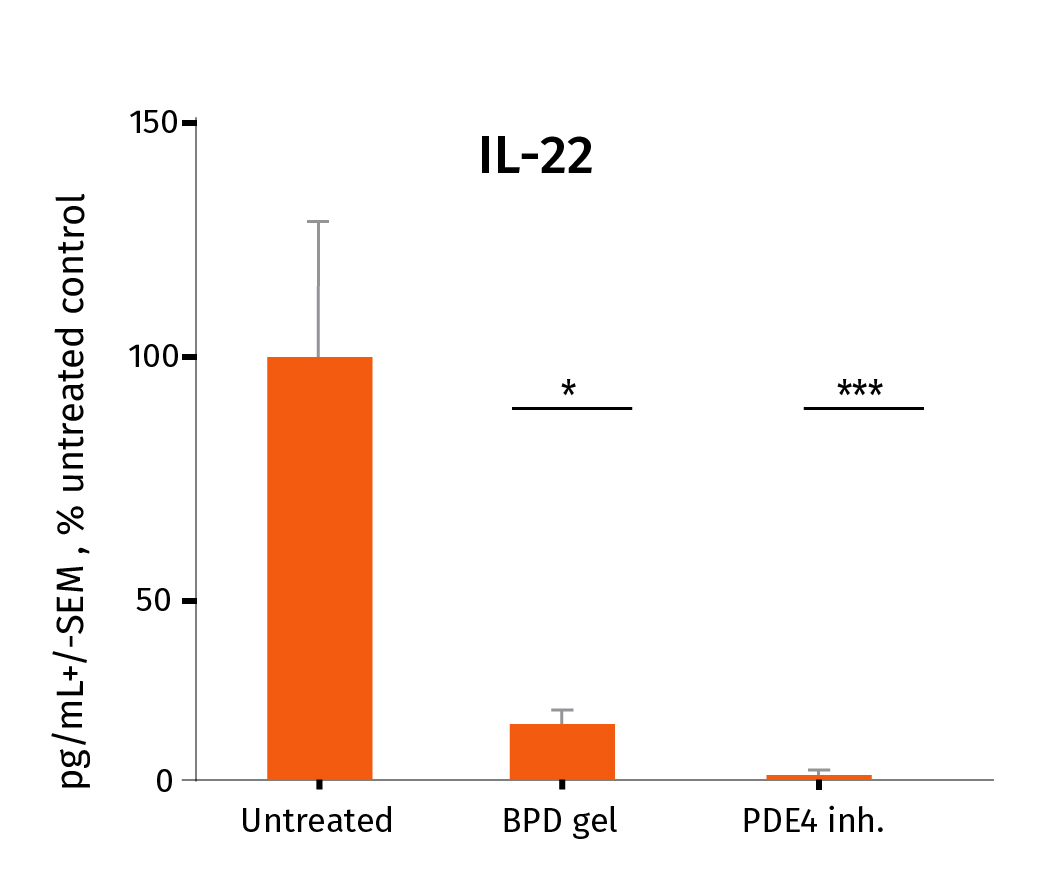
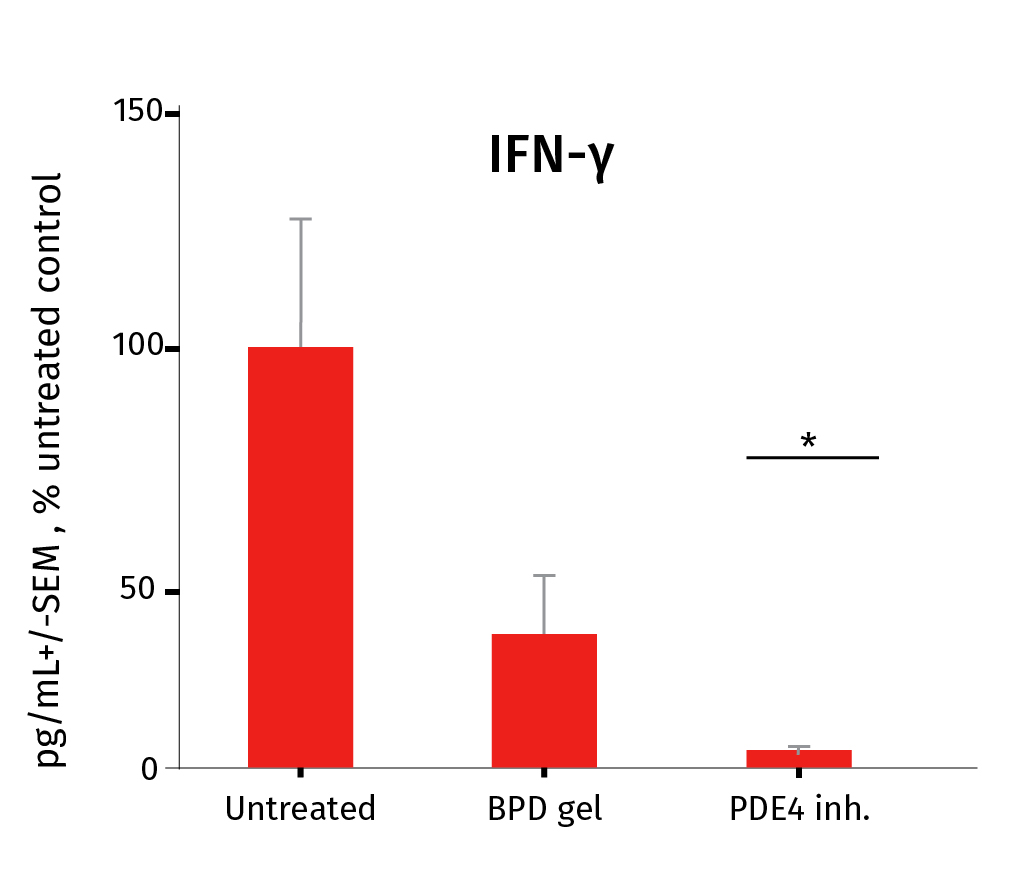
After the experiment, the level of Th17/Th1-related inflammatory cytokines was assessed in the culture medium using our MSD technology for pro-inflammatory markers IL-17A & INF-ᵞ and ELISA for IL-22. The study involved 3 donors. *P<.05, **P<.01, ***P<0.001. The results expressed as the percentage difference compared to untreated control are mean values ± SEM from independent experiments (n=3). A one-way ANOVA statistical test was used to compare treatment effect by BDP and PDE4 inhibitor versus matching vehicle control. For more detailed results don’t hesitate to download the scientific poster.
Study efficacy for multiple donors
Multiple donors to increase study & research relevance
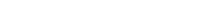
Since our human data generation platforms only contain donated skin, it is possible to conduct studies on multiple donors to obtain relevant and reliable results. The variations in our skin models are donor-dependent and are one of the great strengths of our approach, as they reflect natural biological variations in the general population, allowing for more accurate and relevant study results.

High-quality studies and research on every level
Cutting-edge technology for reliable in-depth analysis

The results obtained from our InflammaSkin® data generation platform are analysed by a team of experts in human skin and its immunology, who work with cutting-edge tools to help you obtain the results that help your research move forward. Our experts master the traditional, standard tests as well as the latest technology to assess skin inflammation, epidermal activation and tissue integrity. We focus on generating unbiased and accurate human data to support safer and faster drug development. Below you’ll find an illustration of our psoriasis research study process.
Scientific publications using our technology & services
See how others use our Inflammaskin® model and services

February 2021 - Calcipotriol/Betamethasone Dipropionate Foam Inhibits Th17 Cytokine Secretion and Improves Epidermal Barrier Markers in a Human Th17 Skin Inflammation Model
- Calcipotriol/Betamethasone Dipropionate Foam Inhibits Th17 Cytokine Secretion and Improves Epidermal Barrier Markers in a Human Th17 Skin Inflammation Model
- Published in Dermatologic Therapy – 2021 Feb;11(1):265-274
- Paola Lovato, Li Jiang, Josephine Hebsgaard, David A Ewald, Hanne Norsgaard
- Published in Dermatologic Therapy – 2021 Feb;11(1):265-274
October 2020 - Development and characterization of a human Th17 driven ex vivo skin inflammation model
- Development and characterization of a human Th17‐driven ex vivo skin inflammation model
- Published in Experimental Dermatology – Oct. 2020;29(10):993-1003.
- Claire Jardet, Anthony David, Emilie Braun, Pascal Descargues, Jean-Louis Grolleau, Josephine Hebsgaard, Hanne Norsgaard, Paola Lovato
- Published in Experimental Dermatology – Oct. 2020;29(10):993-1003.
January 2020 - Translational drug discovery and development with the use of tissue-relevant biomarkers: Towards more physiological relevance and better prediction of clinical efficacy
- Translational drug discovery and development with the use of tissue-relevant biomarkers: Towards more physiological relevance and better prediction of clinical efficacy
- Published in Experimental Dermatology – 2020 Jan;29(1):4-14
- Peter Florian, Klaus R Flechsenhar, Eckart Bartnik, Danping Ding-Pfennigdorff, Matthias Herrmann, Paul J Bryce, Frank O Nestle
- Published in Experimental Dermatology – 2020 Jan;29(1):4-14
October 2019 - Improvements of both inflammation and skin barrier in a human Th17 skin inflammation model by topical treatment with fixed-dose combination calcipotriene/betamethasone dipropionate foam
- Improvements of both inflammation and skin barrier in a human Th17 skin inflammation model by topical treatment with fixed-dose combination calcipotriene/betamethasone dipropionate foam
- Published in Journal of the American Academy of Dermatology – October 01, 2019, Volume 81, Issue 4, Supplement 1, AB79
- Hanne Norsgaard, Paola Lovato, Li Jiang, Josephine Hebsgaard, David Adrian Ewald
- Published in Journal of the American Academy of Dermatology – October 01, 2019, Volume 81, Issue 4, Supplement 1, AB79
September 2019 - Poster - Evaluation of pharmacological responses to subcutaneous injection of adalimumab in InflammaSkin®, a full-thickness human ex vivo skin model reproducing key features of psoriatic lesions
- Evaluation of pharmacological responses to subcutaneous injection of adalimumab in InflammaSkin®, a full-thickness human ex vivo skin model reproducing key features of psoriatic lesions
- Scientific Poster presented at the 2019 Congress of the European Society for Dermatological Research (ESDR) – September 18-21, 2019
- C. Jardet (Genoskin), E. Bartnik (Sanofi-Aventis Deutschland), D. Ding-Pfennigdorff (Sanofi-Aventis Deutschland), E. Braun (Genoskin), P. Descargues (Genoskin)
- Scientific Poster presented at the 2019 Congress of the European Society for Dermatological Research (ESDR) – September 18-21, 2019
May 2018 - Poster - 3D imaging of cleared ex vivo normal human skin, skin appendages and psoriasiform skin lesion using light-sheet microscopy
- 3D imaging of cleared ex vivo normal human skin, skin appendages and psoriasiform skin lesion using light-sheet microscopy
- Scientific Poster presented at the International Investigative Dermatology (IID) 2018 Meeting – 16-19 May 2018
- C. Jardet (Genoskin), S. Abadie (Syntivia), M. Pastore (Genoskin), J. Colombelli (Advanced Digital Microscopy – IRB), B. Chaput (CHU Toulouse Rangueil), A. David (Genoskin), J.-L. Grolleau (CHU Toulouse Rangueil), P. Bedos (Syntivia), V. Lobjois (ITAV), P. Descargues (Genoskin), J. Roquette (ITAV)
- Scientific Poster presented at the International Investigative Dermatology (IID) 2018 Meeting – 16-19 May 2018
September 2017 - Poster - Evaluation of pharmacological responses in a fully human ex vivo skin model following in situ T cell activation with Th17/Th1 psoriasis-like phenotype
- Evaluation of pharmacological responses in a fully human ex vivo skin model following in situ T cell activation with Th17/Th1 psoriasis-like phenotype damage and impairment of protein quality control systems in keratinocytes exposed to a volatile organic compounds cocktail
- Scientific Poster presented at the 47th Annual Meeting of the European Society for Dermatological Research (ESDR) – September 27 to 30, 2017
- P. Lovato (LEO Pharma), C. Jardet (Genoskin), E. Pagès (Genoskin), H. Norsgaard (LEO Pharma), P. Descargues (Genoskin)
- Scientific Poster presented at the 47th Annual Meeting of the European Society for Dermatological Research (ESDR) – September 27 to 30, 2017



