Product spotlight
6
Dec
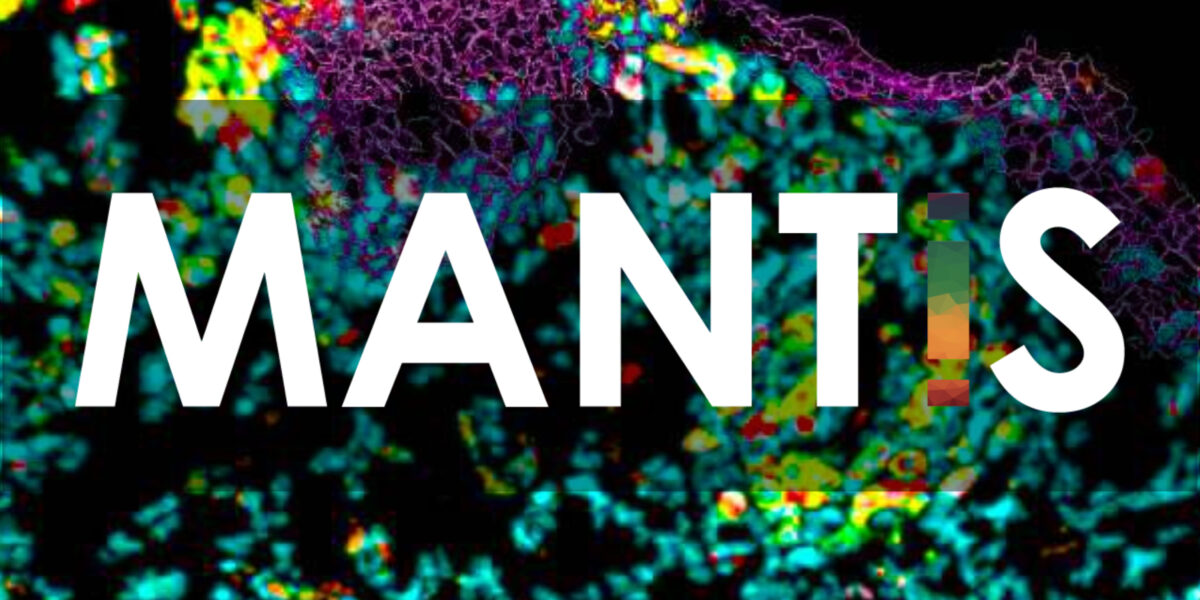
PRESS RELEASE – Genoskin launches spatial skin biology platform MANTIS®
PRESS RELEASE - Toulouse, France & Salem, MA, USA - December 6, 2021 GENOSKIN LAUNCHES SPATIAL SKIN BIOLOGY PLATFORM MANTIS® Genoskin launches MANTIS®, a spatial biology imaging platform dedicated to skin immunology. The MANTIS® (Multiplex ANnotated Tissue Imaging System) platform was developed at Infinity (Inserm U1291, France) by a team of experts under the guidance of Dr. Nicolas Gaudenzio, whoRead more
28
Oct
A unique ex vivo perfused human skin model to test pharmacokinetic and systemic administration in human skin
The FlowSkin® platform : a unique ex vivo perfused human skin model to test pharmacokinetic and systemic administration in human skin When human tissue culture meets microfluidic technology, it leads to the first perfused human skin model ; an amazing platform to overcome the challenges of drug development and replace animal testing. Almost 10 years ago now, Genoskin developed theRead more
4
Sep
PRESS RELEASE – Genoskin launches new offering: NativeSkin access
Genoskin launches new skin offering, NativeSkin® access, that increases accessibility to animal testing alternatives NativeSkin, the only ex vivo human skin model sold online, will be available to purchase daily at a key price point, making it easier to order even for larger volume sales Toulouse, France & Salem, MA, USA, September 1, 2020 - Genoskin, a biotechnology company developing ready-to-use human skinRead more
25
Oct
Genoskin granted new patent on subcutaneous injection model
Genoskin granted a new patent on ex vivo subcutaneous injection model The French National Institute for Intellectual Property (INPI) granted Genoskin's latest patent on its unique ex vivo subcutaneous injection model, HypoSkin®. A boom in the subcutaneous biologics market Today, subcutaneous injection is one of the most common methods to deliver a drug into the human body. Its use has increased significantlyRead more
6
Sep
New study model for injectable anti-psoriasis drugs
New study model for injectable anti-psoriasis drugs A hi-tech psoriasis skin model to study drug efficacy To evaluate the efficacy of injectable anti-psoriasis drugs, the Genoskin team has designed a new test model that combines the InflammaSkin® and HypoSkin® technologies. Below you’ll find a proof-of-concept case study that illustrates the relevance of the new Hypo-InflammaSkin® model during efficacy studies of injectableRead more
15
Feb
Genoskin develops new perfusable vascularized skin model
New ex vivo perfusable vascularized human skin model for drug testing Perfusable vascularized skin for increased viability and optimal skin response Natural human skin contains a rich vascular network, which is essential for the transport and absorption of nutrients and drugs. However, a key drawback of currently engineered human skin models is their lack in vascularization. Recreating a vascularization inRead more