Product spotlight
6
Oct
The Role of Animal-Free Non-Clinical Platforms in Drug Development – Part 1
Redefining Local Toxicity AssessmentPart 1 - The Role of Animal-Free Non-Clinical Platforms in Drug DevelopmentIn the intricate world of drug development, the quest for precision, safety, and efficacy is unending.Traditional toxicity assessment methods using animal models, while historically significant, have shown discernible limitations in their predictability of human responses. As the biotech and pharma sectors continue to push the boundariesRead more
31
May
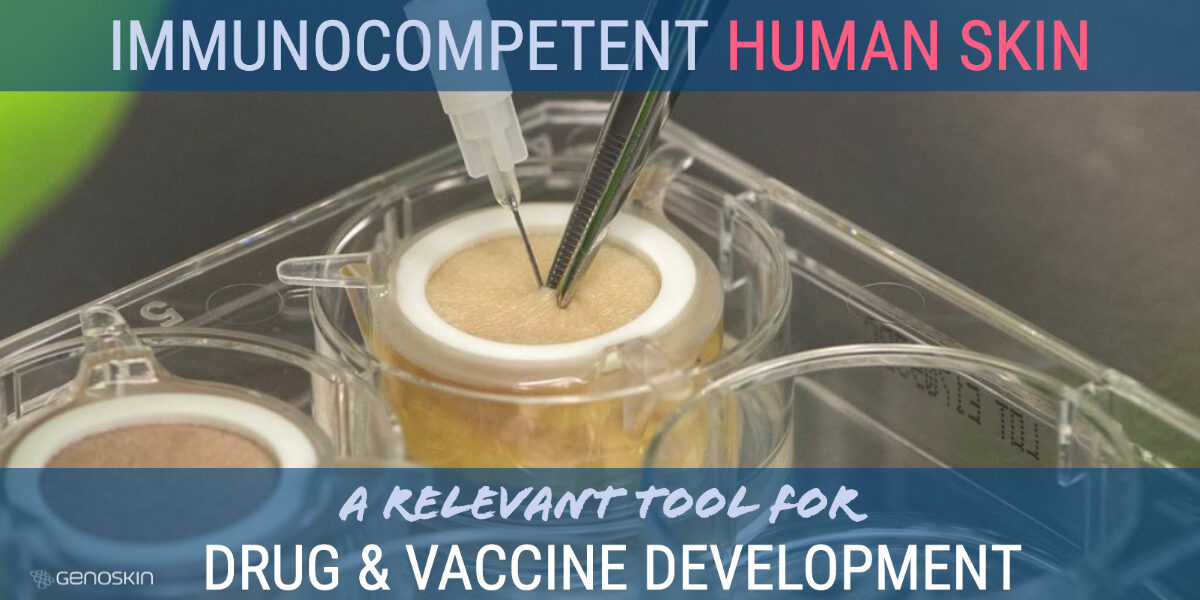
Immunocompetent human skin for vaccine and drug development
Before proceeding to clinical trials in humans, today’s scientists study drug efficacy and toxicity in animals. Even though animal studies clearly raise ethical questions, it is considered even more unethical to conduct studies directly on humans without prior testing. Genoskin provides a unique alternative to animal testing and to human trials, using real, immunocompetent human skin. Why human skin isRead more
6
Dec
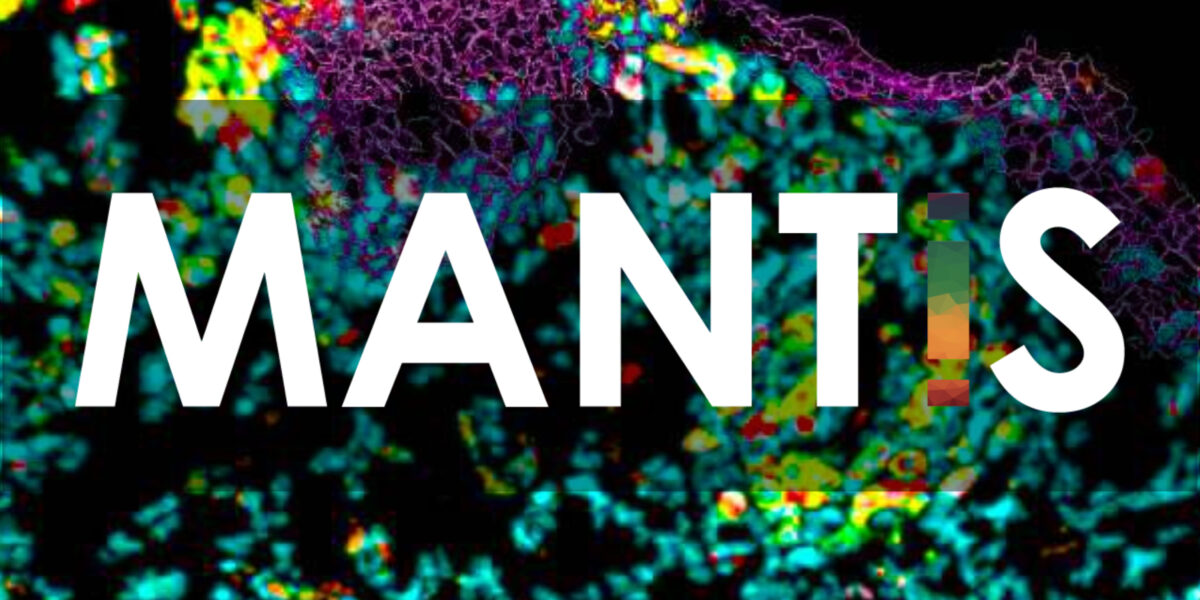
Genoskin launches spatial skin biology platform MANTIS®
PRESS RELEASE - Toulouse, France & Salem, MA, USA - December 6, 2021 GENOSKIN LAUNCHES SPATIAL SKIN BIOLOGY PLATFORM MANTIS® Genoskin launches MANTIS®, a spatial biology imaging platform dedicated to skin immunology. The MANTIS® (Multiplex ANnotated Tissue Imaging System) platform was developed at Infinity (Inserm U1291, France) by a team of experts under the guidance of Dr. Nicolas Gaudenzio, whoRead more
28
Oct
De-risking antiaging trials with ex vivo skin models
Article written by Danny Sullivan and first published in Longevity Technology on October 28th, 2021. The original article is available here. Genoskin can help de-risk antiaging trials with the only live human skin model available for subcutaneous administration testing With its proprietary ex vivo human skin technologies, French partner research organisation Genoskin says it is paving the way to “better,Read more
15
Jun
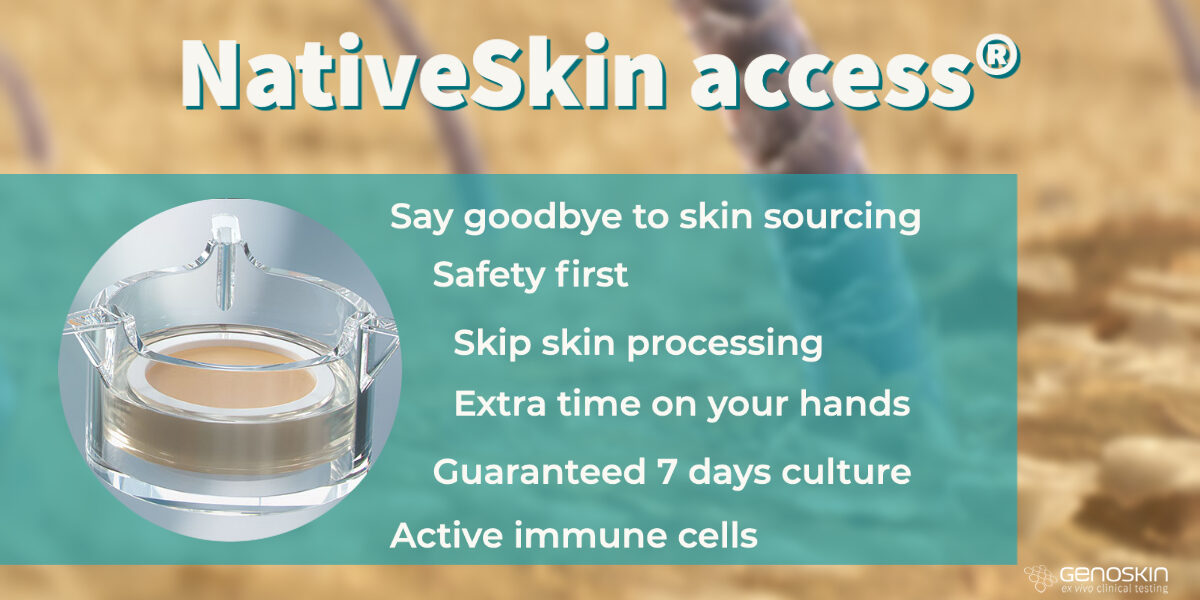
Top advantages of ordering NativeSkin access® vs sourcing skin directly from hospitals
Top advantages of ordering NativeSkin access® vs sourcing skin directly from hospitals Interested in trying NativeSkin access®? Do you still think that sourcing skin samples directly from hospitals is the only valid way? Let us change your mind with these top advantages to ordering NativeSkin access® online! NativeSkin access® is a patented human skin model that makes the culture, treatment,Read more
10
Jun
Injection site reactions platform
ISR PlatformA PROPRIETARY PLATFORM TO TEST INJECTION SITE REACTIONS IN HUMANS AHEAD OF CLINICAL TRIAL.A NEW PROPRIETARY RESEARCH TOOL TO IDENTIFY MAST CELLS-DEPENDENT & INDEPENDENT INJECTION SITE REACTIONS IN HUMAN SKIN. Today, biologics represent one of the fastest growing markets within the pharmaceutical industry. The pipeline of biological drugs is growing at a rapid pace with a global market expectedRead more